Company
Corporate development
Milestones in the history of URSAPHARM.
URSAPHARM will continue to support individuals as a reliable health care partner in the future. In combination with the goal of actively contributing to preserving and improving quality of life.

The year of foundation 1974
The year in which URSAPHARM Arzneimittel GmbH was established. As a distribution company for ophthalmologicals (eye care pharmaceuticals) we lay the foundations for a leading health care company operating throughout Germany and internationally.


Development of the production facilities 1976
URSAPHARM expands its range of services. The development of an innovative production facility for the manufacture of eye drops and eye ointments represents a further milestone along the company’s road to success.


The first modern facility 1979
URSAPHARM optimises its production facilities. A modern facility for the manufacture of solid dosage forms such as granules and tablets is put into operation.


Expansion of the pharmaceutical range 1984
In addition to the manufacture of eye care products, we consolidate our range of pharmaceuticals with the manufacture of general practice products such as mineral supplements, trace elements, products to improve blood circulation and enzymes.


Free from preservatives 1993
With the innovative COMOD® system, URSAPHARM makes it possible to keep liquid dosage forms such as eye drops sterile for long-term use without the addition of preservatives.
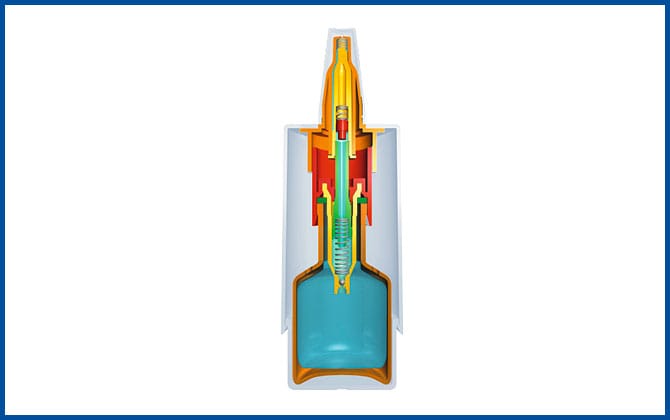
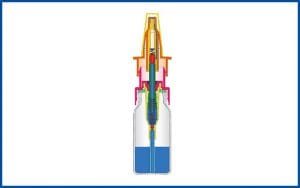
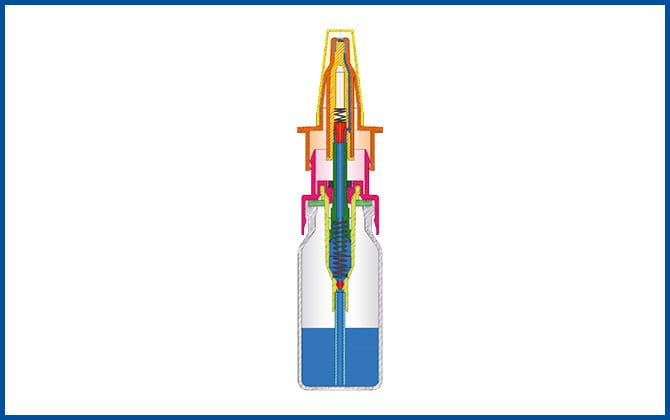
The development of the 3K® pump 1997
The development of the 3K® pump improves the COMOD® system as a non-airless container system and has since proven particularly successful in the manufacture of pharmaceuticals and medical products for nasal use.
HYLO COMOD® 1999
The market launch of the medical product HYLO-COMOD® eye drops was the starting signal for the development of a whole group of hyaluronic acid-containing preparations for the treatment of dry eye.
HYLO GEL® 2008
HYLO®-GEL is the first medical product for eye lubrication in Germany deemed to be prescribable and chargeable to statutory health insurance companies. With this decision, the Federal Joint Committee (Gemeinsame Bundesausschuss, G-BA) confirmed the therapeutic necessity of highly viscous hyaluronic acid solutions for eye lubrication. At the same time, the G-BA identified the absence of a therapeutic alternative to HYLO®-GEL.